FA16 Immunization Module’s Updates
Recent Advances in Vaccine Technology: DNA Vaccines
Advances in recombinant DNA technology, including the development of high-expression multi-gene human vectors (plasmids), have allowed for the potential use of DNA vaccines.
DNA vaccines walk a line between vaccine therapy, immunotherapy, and gene therapy. They consist of circular plasmids containing a high expression promoter (e.g.: CMV, SV40, etc.) coupled to one or more antigens to which immunity is desired. Such plasmids also contain selection markers and can co-express co-stimulatory molecules. Co-stimulatory molecules can also be expressed from accesory plasmids.
Plasmid is delivered by one of two mechanisms: 1) intra-muscular injection with high concentrations of plasmid, or 2) gene gun (nanoparticles coated with plasmid are bombarded at high speed directly into cells).
By a still to be determined mechanism, high concentrations of DNA injected in the extracellular space can result in some partial endocytosis of plasmid and its expression by resident Antigen Presenting Cells (APCs) or myocytes. Lower concentrations of DNA are needed when using a gene gun technique.
Cytosolic expression of antigen results in peptide incorporation and presentation by MHC Class I molecules, sitmulating CD8+ Cytotoxic T Cells (CTL) upon migration of APCs to secondary lymphoid tissue. This behavior is similar to the use of live or attenuated viral vaccines (which can also induce a CTL response), and it is an improvement over protein-antigen vaccines, which are usually restricted to helper T cell and antibody responses only.
DNA vaccines also generate a helper T cell response. Small quantities of antigen generated in myocytes can be expelled into the extracellular space. Secretory forms of antigen can further populate the extracellular space. Expelled and secreted antigen is endocytosed by resident APCs and presented on MHC Class II, which in turn stimulate CD4+ helper T cells upon APCmigration to secondary lymphoid tissue.
The choice of helper T cell response can be intentionally guided towards Th1 or Th2. By a yet to be determined mechanism, intra-muscular injection preferentially induces a Th1 bias, while gene-gun technique induces a Th2 bias. In addition, co-expression of co-stimulatory molecules can bias a Th1 or Th2 response. For example, co-expression of IL-12 supports Th1, while IL-4 supports Th2. Additional co-stimulatory molcules that have been tested include IL-10, IL-15, IL-18, IL-21, CXCL8, CD40, CD40L, IFN-gamma, CD80/B7.1, CD86/B7.2, GM-CSF, and many more. Co-stimulatory molecules help to guide and accentuate the T cell response. Plasmid can also be engineered with high levels of unmethylated CpG dinucleotides, a hallmark of bacterial DNA and potent activator in APCs.
Because antigen is stably expressed for an extended period of time, it can be taken up by follicular dendritic cells and presented to B cells, providing long-lasting humoral immunity, in addition to the aforementioned cellular immunity.
Currently, DNA vaccines have only been approved for veterinary care in the US. Human trials are in progress, particularly for cancer.
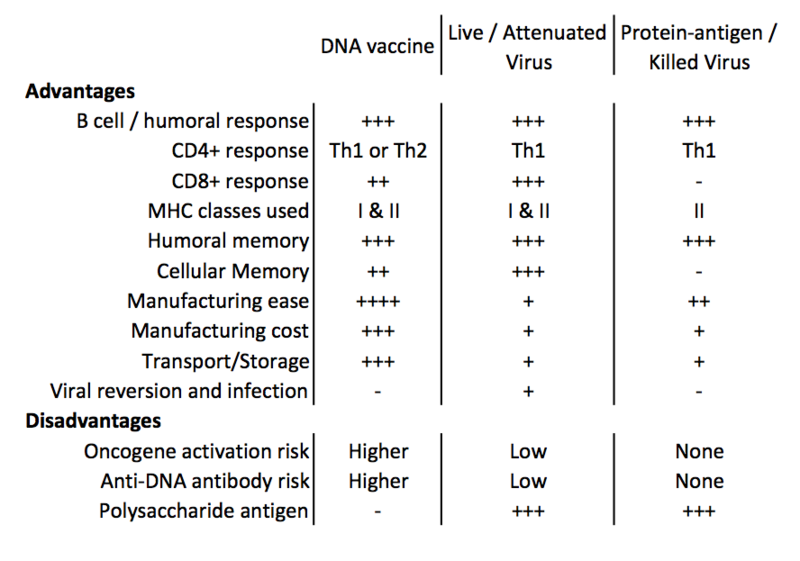
Advantages and disadvantages are summarized below.
1) Tiptiri-Kourpeti, A, et. al. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacology & Therapeutics (2016) 165:32-49
2) Hasson, S, et. al. The past, current and future trends in DNA vaccine immunisations. Asian Pacific Journal of Tropical Biomedicine (2015) 5(5):344-53


@MaryClark, pertaining to DNA vaccines against AchR auto-antibodies in Myasthenia Gravis: for monoclonal antibodies, a DNA vaccine is theoretically feasible. You could isolate the plasma cells producing the one antibody, clone the variable regions (no constant regions), and have that be expressed as peptides on MHC class molecules from the vaccine plasmid. However, in most autoimmune diseases, antibodies are likely polyclonal. You would need a unique vaccine against each auto-antibody. While technically feasible, financially it would be ruinous.
The incidence of cancer is unknown and at this point is purely speculative. Assuming that there is minimal homologous sequence between the DNA plasmid and the endogenous genome, insertion of plasmid DNA should be near zero. And the likelihood that insertion occurs in an oncogenic fashion is also very small. Multiply the two probabilities together, and you end up with an even smaller number. The problem is that, you really only need one truly bad oncogenic insertion in on cell, and that can produce devastating effects.
Scientifically we can say the probability is extremely low, but we can also say it is greater than zero. Patients will care about the latter.
I wonder what kind of incidence there is of cancer after receiving a DNA vaccine. Are we talking 1 in milliion, or 1 in a thousand? What size studies have been done on these vaccines? I'm wondering also if there are other possible effects; what other genes (besides the oncogenes) might be activated?
Also, I wonder if we could extend applications of these vaccines to other kinds of molecular therapies; for instance could we make some kind of vaccine against the antibodies that destroy the ACh receptors in Myasthenia Gravis?
@Arminius Caldwell, I agree that the listed disadvantages involving oncogene activation are an issue, and would also be interested to see if the benefits outweigh the cost... Is the risk only a very small fraction of a percentage increase?
However, I find it especially cool that the manufacturing cost/ease and storage/transport aspects of making DNA vaccines are so much greater, I'd be interested to see what the public health impact is on disadvantaged communities that can't regularly afford large quantities, or--even better--the impact on communities that were previously considered inaccessible due to geographical and infrastructure limitations.
1. Gene gun sounds like the coolest procedure to run, I hope I get the chance to do it one day.
2. The biggest thing that jumps out to me on that chart is the increased risk of cancer vs the efficiency of manufacturing and storage. I wonder if the human trials will find the cancer risk low enough to be viable as a treatment.