FA16 Immunization Module’s Updates
Recent Advances in Vaccine Technology: Novel Adjuvants
Currently, there are a limited number of approved adjuvants available for vaccine formulation. These include alum, MF59 (oil/water emulsion), AS03 (oil/water emulsion), a virosome, and AS04 (alum-adsorbed TLR4 agonist). Alum has been in use since 1926, while AS04 is the newest approved adjuvant and has been in use since 2005 [1]. While these have sufficed thus far for the vaccinations normally administered throughout life, a 2015 article from Immune Network points out that, “new vaccine targets require the induction of well-defined CMI [cell-mediated immunity] in addition to high titer of antibody. Consequently, new immunostimulant adjuvants in vaccine formulations are needed in order to stimulate robust immune responses including humoral immunity and CMI” [1].
The article details several classes of adjuvants that are undergoing clinical trials. TLR (toll-like receptor) agonists are one of the subsets being tested. As Lee and Nguyen state, “TLRs provide a bridge between innate and adaptive immunity,” so TLR agonists as adjuvants have the potential to provide a full immune response as well as a response that is more selectively targeted than one elicited by, for example, alum [1]. There are three TLR agonist adjuvants mentioned in the paper as undergoing clinical trials: CpG, which, unsurprisingly, is composed of CpG oligonucleotides and is a TLR 9 agonist; flagellin (linked to the antigen), a TLR 5 agonist; and PolyI:C, composed of dsRNA analogues and is a TLR 3 agonist [1]. Thus far in their testing, these adjuvants have been shown to enhance antibody titer as well as successfully stimulate the TLR signaling pathway, causing release of several proinflammatory cytokines and subsequent stimulation of CMI [1].
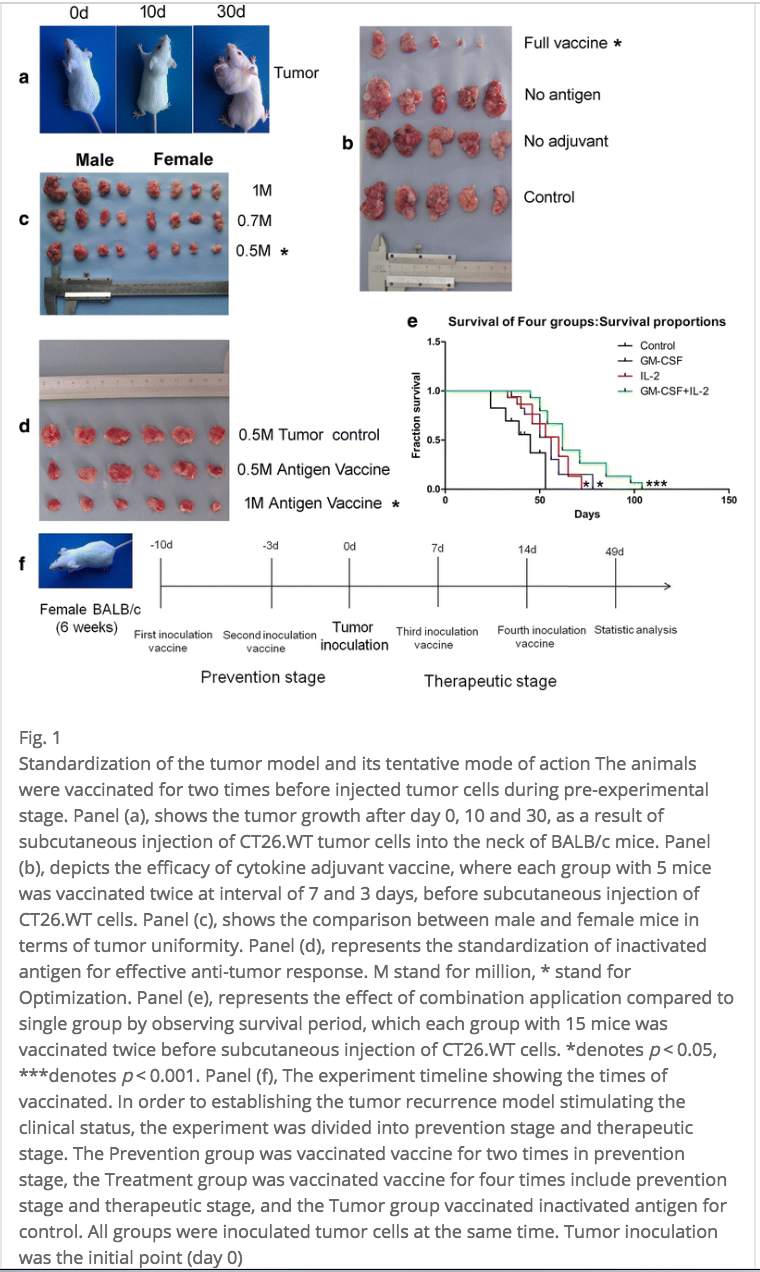
Some researchers are investigating the use of cytokines themselves as adjuvants. A paper published just a few days ago in BMC Immunology reports cautiously promising results with use of a cytokine adjuvant in an immunotherapy treatment for colon cancer. They used the cytokines GM-CSF and IL-2 in their vaccine. Among other things, they measured tumor growth and survival in mice injected with CT26.WT (colon cancer cells), and found that tumor growth was “significantly inhibited” and the survival period for vaccinated mice was at least 160 days longer than that of unvaccinated mice [2].
Below is a nice picture from their experiment—if you look at panel B, you can see all the tumors resected from the mice in that particular portion of the experiment. The sample size in each group was quite small, so hopefully these results are replicable on a larger scale!
[1] Lee, Sujin, and Minh Trang Nguyen. 2015. “Recent Advances of Vaccine Adjuvants for Infectious Diseases.” Immune Network 15.2: 51–57.
[2] Ju, H. et al. 2016. “An effective cytokine adjuvant vaccine induces autologous T-cells response against colon cancer in an animal model.” BMC Immunology 17:31.


Great post! I am still confused as to why the United States primarily uses aluminum salts and aluminum gels as adjuvants when we know the European model of using monophosphoryl lipid A and MF59 are much more effective at delivering vaccines? I wonder if this has anything to do with the tentacles of big pharma reaching into the government and dictating policy?
Exciting use of cytokines as adjuvants. It's good to hear that they are looking into better adjuvants since there is some research that aluminum in adjuvant form could cause immunological risks, risks for long-term brain inflammation and associated neurological complications according to the below.
https://www.ncbi.nlm.nih.gov/pubmed/21568886
Great article! I never really considered the depth of thinking that goes into the creation of adjuvants. I appreciate that you included very up to date research, seems like an interesting field to be in right now.