FA16 Immunization Module’s Updates
Vaccine Storage and Handling
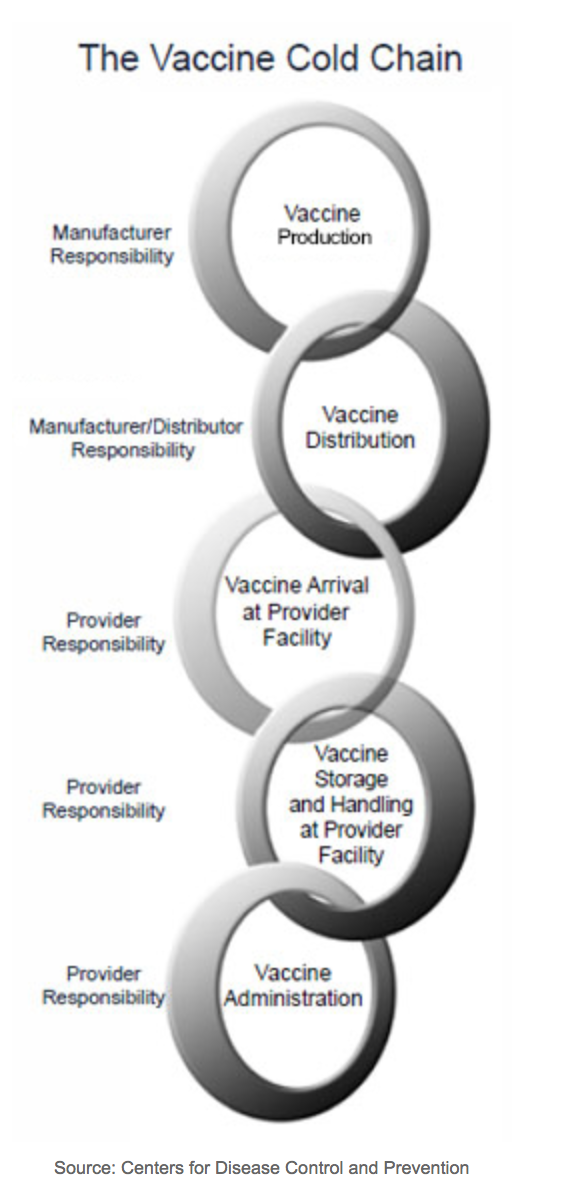
Vaccine storage and handling is critical because it can reduce vaccine potency leading to poor protection against disease. Improper storage and handling of vaccines can also have a financial impact. Vaccine storage and handling errors can occur at various intervals ranging from the time the vaccine is manufactured to the time it is administered. The major component of handling and storage of a vaccine is temperature. Excessive heat, cold, or light can exposure can reduce the potency of vaccines. The temperature-controlled environment used to maintain and distribute vaccines in optimal condition is called the vaccine cold chain (Image below).
There are three key components that providers need to store and handle vaccines appropriately. These include written vaccine storage and handling plans, a well-informed staff, and proper storage equipment. A written vaccine storage and handling plan should include information in regards to storing vaccines, ordering vaccines, and storage and handling of vaccines in emergency events (freezer malfunctions, power failures, natural disasters, etc.). Staff members and any person who has access to vaccines must be informed about storage and handling of vaccines. Also, one staff member should be given the responsibility as the primary individual who handles and cares for vaccines. Vaccine storage equipment must be properly maintained and ensured to be at the correct temperature. Vaccines requiring storage temperatures between 2°C and 8°C should be stored in a stand-alone refrigerator and vaccines requiring storage temperatures between -50°C and -15°C should be stored in a separate stand-alone freezer. Vaccines sensitive to light can be kept in their original packaging, which also helps with inventory management and keeping track of expiration dates. Each vaccine's storage and handling recommendations are included in the manufacturer's product information and vaccines past their expiration should NEVER be used.
Resources: You Call the Shots" web-based immunization training course module from the CDC


According to the resource varicella-containing vaccines should be stored in a freezer as they can deteriorate quickly. According to my research it seems live vaccines are more susceptible to heat and inactivated vaccines are actually damaged by freezing. One example of a live vaccine which does not have to be frozen is the MMR vaccine. You can check out this pdf link for more information particularly the section titled Refrigerator or freezer.
http://www.immunize.org/guide/aov04_storage.pdf
This reminds me of handling enzymes and other reagents in the lab. In the lab, we stored enzymes, DNA, and RNA in -20C or -80C. The reagents considered most unstable (e.g., RNA and enzymes) were stored at colder temperatures. My guess is that the live vaccines are actually kept at colder temperatures. Any ideas/answers @Muhammad Ilyas ?
I find it interesting that there are some vaccines that need to be stored at temperatures below freezing; @Muhammad Ilyas do you know which vaccines these are? I would assume it cannot be any of the live vaccines, since those would likely be killed by the freezing temperatures.