FA16 Immunization Module’s Updates
Storage of Vaccines
Depending on the vaccine to be used, there are different guidelines set out by the Center of Disease Control (CDC). The most fragile of vaccines that must be have special attention are live attenuated vaccines. These live vaccines are derived from disease-causing viruses or bacteria. Before they are administered in treatment, they are attenuated, or weakened, in a laboratory. Although live attenuated vaccines will begin to replicate in humans when administered in treatment, they the disease state is much milder compared to the natural disease state. These types of vaccines are extremely fragile because they have been taken out of their native state and mutate. As a result, proper handling must ensure that the vaccines remain out of high heat or light. Examples of live attenuated vaccines include measles, mumps, and rotavirus.
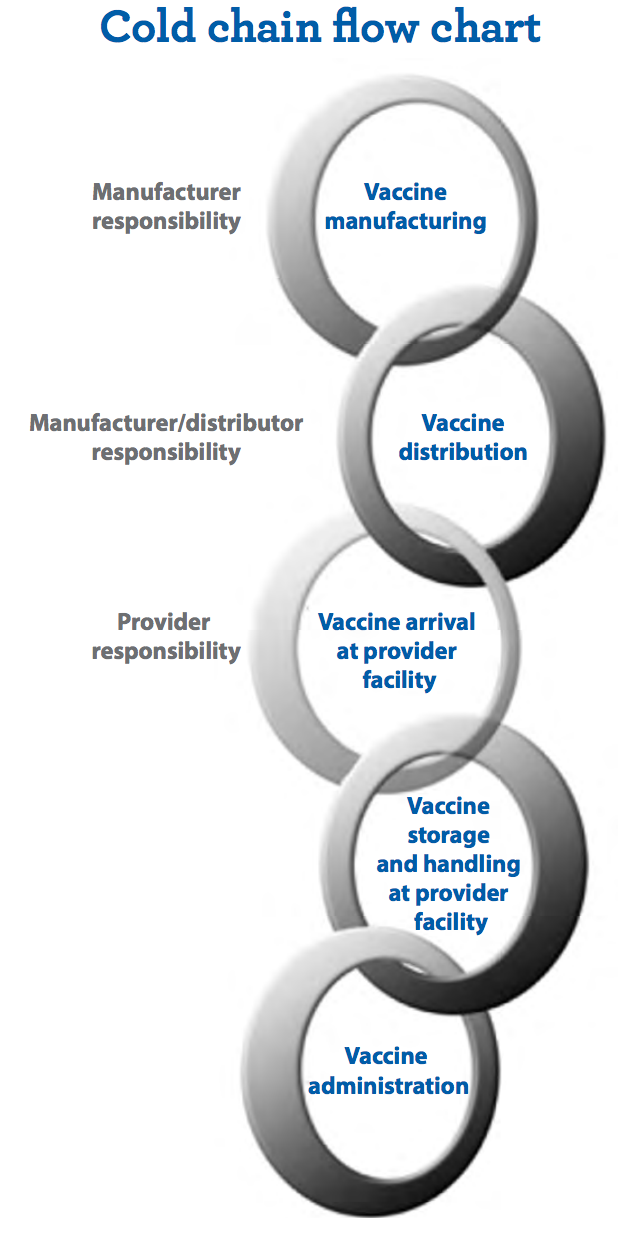
For other less fragile vaccines, the CDC recommends a series of guidelines for referigeration or freezing. Importantly, they have come up with a "Toolkit" as a means of outlining how health care organizations should distribute and maintain vaccines. The general outline is called a "Cold chain flow chart." This chart as shown below highlights each stage of vaccine production from manufacturing to administration.
At each of these stages, the CDC ensures that the vaccine to be produced is well-insulated so that it will not degrade.
Finally, since vaccines are particularly important for young children, the American Academy of Pediatrics has come up with their own table and chart for vaccine storage. These guidlines are to be maintained through hospital systems to ensure that vaccinations can be performed consistently and efficiently. Ultimately, the production of vaccines is a multi-step process that involves a series of checkpoints to ensure that the vaccine can be fully functional by the time of administration. Depending on the type of vaccine used, there can be different guidelines outlined by the CDC.
Resources:
http://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storage-handling-toolkit.pdf
http://www.cdc.gov/vaccines/pubs/pinkbook/prinvac.html
http://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storage-handling-toolkit.pdf
https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/immunization/Pages/vaccine-storage-chart.aspx?nfstatus=401&nftoken=00000000-0000-0000-0000-000000000000&nfstatusdescription=ERROR%3a+No+local+token


I found this information to be very interesting. I think that it is indeed important to have proper storage and handling of vaccines. If this is not properly done, then this can result in a decreased effectiveness of the vaccine. I think that it is important to have different checkpoints and stages of storing vaccines in order to prevent potential errors once they are given to patients. I did not know that there was a special protocol in storing vaccines for children.